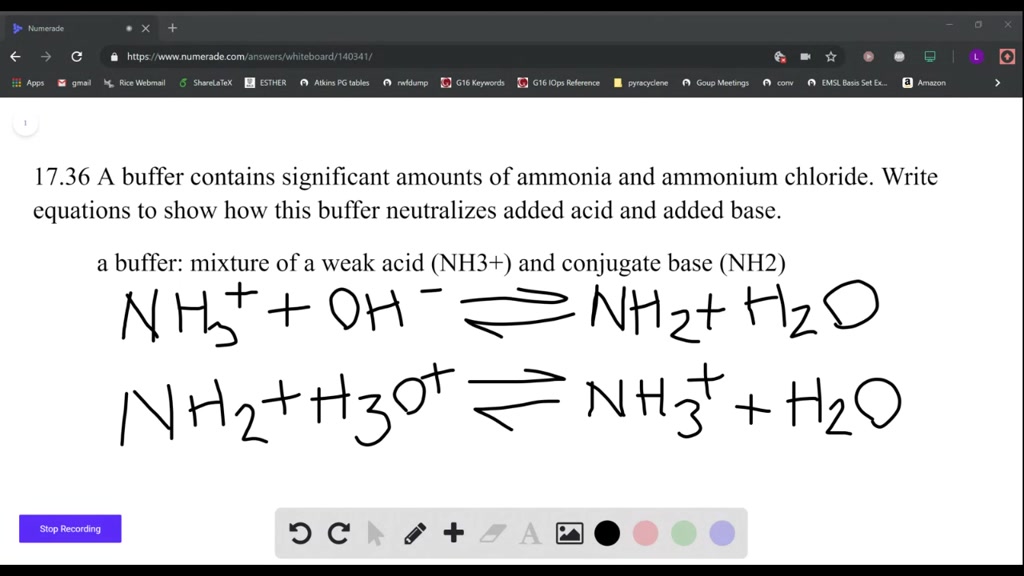
Is Nh4cl A Conjugate Acid
When discussing acids and bases, one important concept to understand is conjugate acids and bases. In this article, we will explore the question: Is Nh4cl A Conjugate Acid? To answer this question, we must first delve into the properties of Nh4cl and its behavior in aqueous solutions.
Nh4cl, also known as ammonium chloride, is a compound made up of ammonia (Nh4) and hydrogen chloride (HCl). When Nh4cl is dissolved in water, it undergoes dissociation to form ammonium ions (Nh4+) and chloride ions (Cl-). This process results in the formation of an acidic solution due to the presence of the ammonium ion.
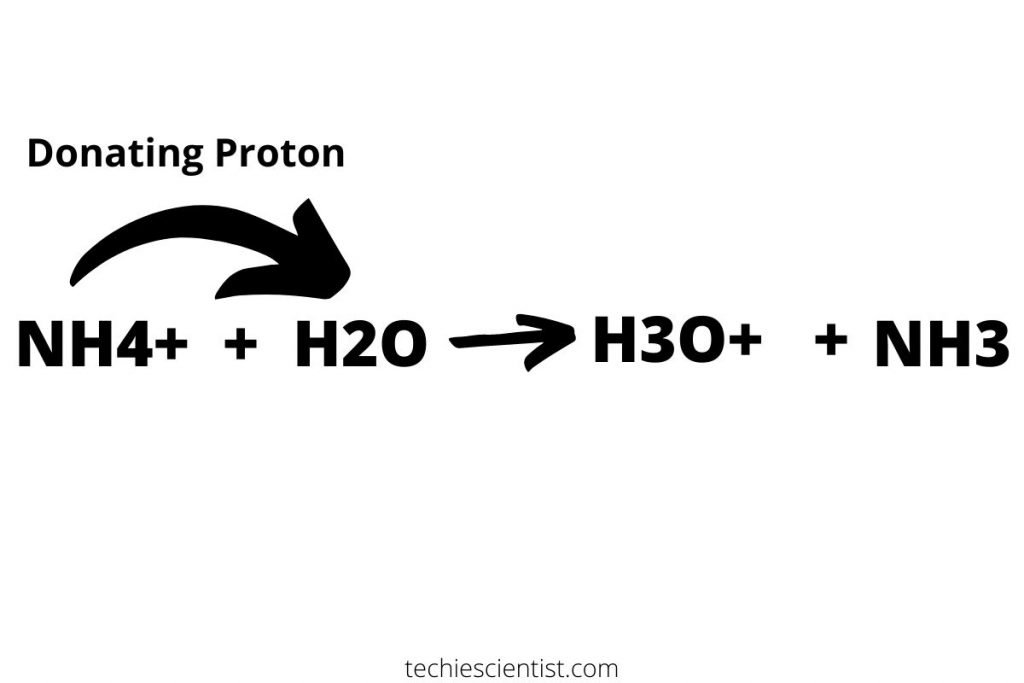
So, is Nh4cl a conjugate acid? To answer this question, we must look at the behavior of Nh4cl in water. When Nh4cl is dissolved in water, the ammonium ion (Nh4+) can act as a weak acid by donating a proton to water molecules. This donation of a proton results in the formation of the conjugate base, ammonia (Nh3), making Nh4cl a conjugate acid.
Understanding Conjugate Acids and Bases
What are Conjugate Acids and Bases?
Conjugate acids and bases are pairs of compounds that differ by a single proton (H+). When an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid. This relationship allows for the transfer of protons between molecules, resulting in the formation of different species with varying acidic or basic properties.
How are Conjugate Acids and Bases Related?
The strength of a conjugate acid-base pair is inversely related. This means that a strong acid will have a weak conjugate base, and vice versa. Similarly, a strong base will have a weak conjugate acid, and a weak base will have a strong conjugate acid. Understanding the relationship between conjugate acids and bases is essential in predicting the behavior of acidic and basic solutions.
Properties of Nh4cl as a Conjugate Acid
Acidic Properties of Nh4cl
As mentioned earlier, Nh4cl exhibits acidic properties when dissolved in water. The presence of the ammonium ion (Nh4+) allows Nh4cl to act as a weak acid by donating a proton to water molecules. This donation of a proton results in the formation of the conjugate base, ammonia (Nh3), which can then accept a proton to reform the original acid, Nh4+.
Amphoteric Nature of Nh4cl
Nh4cl also demonstrates amphoteric behavior, meaning it can act as both an acid and a base depending on the chemical environment. In the presence of strong bases, Nh4cl will behave as an acid by donating a proton, while in the presence of strong acids, it will act as a base by accepting a proton. This dual nature of Nh4cl highlights its versatility in different chemical reactions.
Conclusion
In conclusion, Nh4cl can be considered a conjugate acid due to its ability to donate a proton and form the conjugate base, ammonia. Understanding the properties of Nh4cl as a conjugate acid is essential in the study of acid-base chemistry and the behavior of acidic solutions. By exploring the relationship between conjugate acids and bases, we can gain a deeper insight into the nature of chemical reactions involving acids and bases.
Mays Landing Loretta Burroughs Nj
Discovering Isobel Laidler: A Rising Star In The Industry
Jane Chuck Biography Real Name

ncG1vNJzZmicpae2r3rApqpsZpSetKrAwKWmnJ2Ro8CxrcKeqmebn6J8qr%2BMp59tm5xirm6vzqehrp%2BRqbJurcKim2egpKK5